What Should Be The Cut Off Of Inbreeding Coefficient In Registered Angus

Parentage-Based Group Composition and Dispersal Design Studies of the Yangtze Finless Porpoise Population in Poyang Lake
1
The Primal Laboratory of Aquatic Biodiversity and Conservation of the Chinese Academy of Sciences, Establish of Hydrobiology of the Chinese Academy of Sciences, Wuhan 430072, Red china
ii
Research Center of Aquatic Organism Conservation and Water Ecosystem Restoration in Anhui Province, School of Life Sciences, Anqing Normal University, Anqing 246133, China
3
University of the Chinese Academy of Sciences, Beijing 100049, Red china
*
Authors to whom correspondence should be addressed.
Academic Editor: Li Lin
Received: 23 May 2016 / Revised: xvi July 2016 / Accustomed: 28 July 2016 / Published: 11 Baronial 2016
(This article belongs to the Section Biochemistry)
Abstract
Social behaviors are poorly known for the critically endangered Yangtze finless porpoise (YFP, Neophocaena asiaeorientalis asiaeorientalis). Here, grouping composition and dispersal patterns of the YFP population living in the Poyang Lake were studied by parentage-based pedigree analyses using 21 microsatellite loci and a 597 bp segment of the mitochondrial Dna control region. In this study, 21 potential mother-offspring pairs and six potential father-offspring pairs (including two potential parents-offspring pairs) were determined, among which 12 natural mother-offspring groups and a maternal group of iii generations were plant. No genetically-determined fathers were institute associated with their offspring. This study also found that maternally related porpoises at the reproductive state tend to grouping together. This suggest maternal relationship and reproductive state may be factors for grouping in the YFP population. In natural mother-offspring groups, male person offspring were all younger than ii years sometime, which propose male offspring may leave their mothers at approximately ii years of age, or at least they were not in tight association with their mothers equally they may have been under two years old. However, female offspring can stay longer with their mothers and tin can reproduce in the natal group.
1. Introduction
The social behavior of cetaceans is both complex and interesting. Advances in molecular techniques have led to an increasing number of studies that combine molecular, observational, and photo-ID information to reveal a variety of group and dispersing patterns in cetacean species [1,2]. Various cetacean social behaviors have been reported, including fluid fission-fusion societies described in pocket-size delphinid species (e.grand., bottlenose dolphins (Tursiops aduncus) [3]; spinner dolphins (Stenella longirostris) [4]), matrilineal groups in larger toothed whales (eastward.g., killer whales (Orcinus orca) [five]; sperm whales (Physeter macrocephalus) [6]), and associations among individuals of both sexes or just a single sex that vary in size (from few to hundreds of individuals), duration (temporary or permanent), and limerick (single or multiple generations) of Atlantic white-sided dolphins (Lagenorhynchus acutus) [7]. The social behavior of freshwater dolphins was also observed. Smith and Reeves [eight] reviewed that Amazon River dolphins (Inia geoffrensis) sometimes grade loose fishing groups and male-on-male assailment is common. Irrawaddy dolphins (Orcaella brevirostris) course fission-fusion group dynamics with frequent social interactions and cooperative feeding, and mother-young associations can exist observed in the Ganges and Indus dolphins (Platanista spp.). Still, compared to marine cetaceans, knowledge well-nigh the social behavior of freshwater cetaceans remains very express.
The Yangtze finless porpoise (YFP, Neophocaena asiaeorientalis asiaeorientalis) is a small-scale, freshwater toothed whale that occurs merely in the middle and lower reaches of the Yangtze River (from Yichang to Shanghai) and its bordering lakes (Poyang and Dongting) [9]. Due to its pocket-size population size, sharply declining population, and high probability of extinction, the YFP was recently reclassified a Critically Endangered (CR) population in the International Wedlock for the Conservation of Nature (IUCN) Ruby List of Threatened Species [x,11]. Since the YFP is notoriously difficult to identify and track using normal observation methods (e.grand., visual surveys and photo-identification), primarily due to their small size (≈1.five m in length), lack of a dorsal fin, and behavior (e.thou., only surface for 1–ii s at a time), petty is known about their group composition and dispersal patterns. Agreement the social behaviors of the YFP will be instrumental for effective conservation and contribute to our full general knowledge of the social behaviors of freshwater cetaceans [12,13,14].
Previous research suggests the YFP in the Yangtze main stream alive either alone, or in groups of 2–20 individuals [15,16]. Groups consisting of two to three individuals have been suggested to be the most common and are generally called "core units". Groups of >20 individuals are rare and likely consist of several core units. Similar group patterns have besides been reported for porpoises living in semi-natural reserves [17,xviii,nineteen]. Due to the limits of observational data, none of these previous studies take analyzed genetic relationships among individuals inside YFP groups, nor investigated dispersal patterns.
Poyang Lake is the most important limnic habitat of the YFPs with a population of ≈450 individuals (well-nigh 50% of the total population of the YFPs [20]). To investigate the wellness and social structure of the Poyang Lake population, iv capture-release surveys were conducted in the leap of 2009, 2010, 2011, and 2015, during which all captured individuals were marked with an internal ID and genetic samples were obtained. This dataset offers a unique opportunity to study aspects of social behaviors using both genetic and observational information. In this study, we used observational data nerveless during these four capture surveys and genetic data obtained from 21 microsatellite loci and a 597 bp highly variable segment of the mitochondrial Dna control region, to study the relationships of individuals in natural groups and the dispersal patterns of the YFP population living in the Poyang Lake.
2. Results
two.1. Genetic Variation
A total of 171 alleles were detected at 21 microsatellite loci amid 122 individuals. No testify was constitute for null alleles, stuttering and allele dropout in each locus by the program MICRO-CHECKER, at a confidence level of 95%. The number of alleles (N a) ranged from four to sixteen (mean eight.ane), with observed heterozygosity (H o) ranging from 0.374 to 0.854 (mean 0.661) and expected heterozygosity (H e) ranging from 0.361 to 0.828 (mean 0.674; Table ane). The polymorphic information content (PIC) ranged from 0.345 to 0.801 (mean 0.629; Tabular array 1). The combined non-exclusion probability for one candidate parent (Due north e-1p) and for one candidate parent given the genotype of a known parent (North eastward-2p) were vii.20 × 10−4 and 2.17 × 10−half-dozen, respectively (Table ane). In other words, the full exclusion probability of the combined loci when no parents were known (PE 2) was 99.93%, and when one parent was known (PE I) was 99.99%. No deviation from Hardy-Weinberg equilibrium was detected for each locus. The inbreeding coefficient index (F is) of each locus ranged from −0.182 to 0.178, with an boilerplate of 0.0002 (p > 0.05).
Amongst the 122 individuals, three mitochondrial DNA (mtDNA) haplotypes were found: NAACR-Hap1, NAACR-Hap2, and NAACR-Hap8 (GenBank accretion number: KC135874, KC135875 and KC135881). NAACR-Hap1, NAACR-Hap2 and NAACR-Hap8 were shared past 53, 64, and 5 individuals, respectively (Table S1).
two.2. Parentage Consignment and Relatedness
Twenty-one potential mother-offspring pairs and half dozen potential father-offspring pairs (including two potential mother-father-offspring families: 2015F11-2015M3-2015F12, 2009F7-2009M2-2009F8) were detected by CERVUS (Figure 1).
Among the detected female parent-offspring pairs, x female person offspring ranging 0.1–9.five years erstwhile, were found associated with the mother co-ordinate to the field observation record, of which four were aged >two years old (Figure one, Table S2), while vi male calves ranging 0.3–1.seven years sometime were found associated with the female parent (Tabular array S2). Two female individuals, 2015F2 (5.2 years erstwhile) and 2015F10 (1.8 years old), and three male individuals, 2009M14 (six.8 years old), 2015M8 (5.4 years old), and 2011M6 (3.vii years erstwhile), were institute not associated with the mothers (Tabular array S2). All mothers and their offspring shared the same mtDNA haplotype (Table S2). No genetically-determined fathers were institute associated with their offspring (Table S2). The mean pairwise relatedness alphabetize (r) value for the potential mother-offspring pairs was 0.4479 (0.2924–0.5551), and for the potential male parent-offspring pairs was 0.3863 (0.2295–0.5032) (Figure one). The mean overall r value amongst all adults and amid all individuals was 0.039 and 0.040, respectively. In full general, individuals with relatedness values larger or equal than half-siblings (one-half-sibs) (the theoretical r ≥ 0.25) are accounted related, while all other individuals remain unrelated (the theoretical r < 0.25) [21]. Even so, in natural populations, the r values estimated from microsatellite loci may fluctuate around the theoretical value. Blouin et al. [22] suggested using the midpoint betwixt the means of the two distributions equally the cut-off value for nomenclature. For example, if 1 wanted to distinguish between half-sibs (the theoretical r = 0.25) and lower form related individuals (the theoretical r = 0.125) in a natural population, the cut-off value should be 0.1875. That means individual pairs with r ≥ 0.1875 would be classified every bit belonging to the category whose relatedness is larger than half-sibs. Using the cut-off values of r ≥ 0.1875, we institute that 4.48% of all pairs of individuals were related to some extent.
2.3. Composition of Natural Groups
During all 4 capture-release surveys (particularly those conducted in 2009 and 2010 in the channel), human disturbance due to the use of chasing boats and racket may have separated or centralized porpoise individuals. Therefore, nigh big groups captured in a single net were unlikely to be natural groups. In 2011 and 2015, the survey was conducted in sandpit areas. No chasing boats were used. Hence, human disturbance and sampling bias were largely reduced. We were fortunate plenty to find a maternal line consisting of 3 generations in a single group captured in 2011 (Figure one). There are iv mother-offspring pairs detected in this maternal line, and all members in this maternal line shared the same mtDNA haplotype (NAACR-Hap2; Table S1). Based on the detected genetic relationship and the observation data which showed that they grouped together (Tabular array S2), we treated members in the maternal line as a natural group. Additionally, twelve mother-offspring groups (groups 2015F2-2015F1, 2015F11-2015F12, 2015F19-2015F20, 2011F2-2011F1, 2011F4-2011M3, 2011F12-2011F10, 2011F23-2011F22, 2011F24-2011M19, 2011F25-2011M20, 2010F11-2010F12, and 2009F7-2009F8, Group 2009F7-2015M7) were further detected (Figure 1). These were all considered to be natural female parent-offspring groups based on a high caste of behavioral interaction (field ascertainment data), and genetic relatedness.
3. Give-and-take
3.i. Parentage and Relatedness
When using microsatellite markers for paternity analysis, the credibility of the results is highly dependent on the exclusion probability [fourteen,23]. As revealed past previous studies, paternity results are apparent when PE I and PE II values exceeded 99.nine% and 99%, respectively [14,23,24,25,26]. In this report, PE I and PE II values were 99.99% and 99.93%, respectively, indicating that the 21 microsatellite loci we used were advisable for parentage analysis. This was as well supported by the fact that nearly all suspected mother-offspring pairs (those observed together and captured in one net) were genetically assigned mother-offspring status (Effigy ane, Table S3).
In contempo years, serious human interference within the estuary area of the Poyang Lake (e.g., the construction of ii bridges beyond the outlet area, and numerous sand-transport vessels) has caused the river-lake migration of the YFPs to be largely restricted. Thus, inbreeding within the Poyang Lake population has get a matter of great concern. The genetic multifariousness of this population, with a mean H o = 0.661 and a hateful H e = 0.674, is moderate compared to other cetaceans [27,28]. Our relatedness analysis revealed that there is no genetic signature of inbreeding in the YFP population living in Poyang Lake. Outset, no meaning F is was detected in this study. 2nd, our relatedness index did not reflect inbreeding. In a free-range population, the boilerplate r betwixt potential parents should be close to zero (due east.grand., −0.014 in Ursus arctos, [14]; 0.056 in Ursus americanus, [13]), and we found that the mean r betwixt potential parents was very low (0.039). Third, as reported by Csilléry et al. [21], for natural populations, nosotros found that the ratio of private pairs with some relatedness was <ten% (4.4%), further indicating little inbreeding in this population currently. Conversely, the population in Tian'ezhou ex situ reserve demonstrates 26.fourteen% of individual pairs have some relatedness (r > 0.1875) [29]. Chen et al. [29] inferred this ex situ population was probably in high risk of, or has already been suffering from, inbreeding.
3.two. Group Composition
Groups equanimous by maternal human relationship are very common in marine odontocetes (east.1000., long-finned pilot whales (Globicephala melas) [30]; belugas (Delphinapterus leucas) [31]; sperm whales (Physeter macrocephalus) [32]; killer whales (Orcinus orca) [33]). For case, adult female person sperm whales associate with sub-adults to form cohesive 'social units' that can remain together over several years [32]. Similarly, killer whales are besides characterized either by multi-matrilines (resident killer whales [33]) or a single matriline (transient killer whales [5]). In this study, we also detected a maternal line in i YFP group living in the Poyang Lake. Individuals 2011F19, 2011F20, 2011F21, 2011M15, and 2011M16 were found to exist grouping together and were afterward captured in one net. Genetic results showed that this group comprised of four mother-offspring pairs belonging to one maternal linage. The oldest female, 2011F19, is at the acme of the maternal linage and female parent to two other adult females (2011F20, 2011F21) and a calf (2011M15), and grandmother to calf 2011M16, and the offspring of female 2011F21 (Effigy 2). Furthermore, female person 2011F20 was confirmed pregnant through B-type ultrasonic inspection. The matrilineal grouping design of the YFPs is further supported by the structure of another group (including individuals from 2009M1 to 2009F3) (Table S3). Groups captured in 2009 cannot exist treated every bit natural groups considering sound chasing operations during the survey may have disturbed the natural behavior in these porpoises. Notwithstanding, our genetic results revealed that adult females 2009F1, 2009F2, and 2009F3 in this group were very closely related, with r values close to that of full-sibs (0.4012 to 0.5372). As these iii females shared the same mtDNA haplotype (Table S1), nosotros infer that they have high matrilineal relationship. B-type ultrasonic inspection further confirmed these females were all pregnant.
Information technology is interesting that maternally related female YFPs grouping together were all in various reproductive stages. This phenomenon has also been found in other cetacean species, where females with a dependent calf often course nursing groups to reduce unpredictable risk [34]. Indeed, females may class loose associations with related or unrelated females, preferentially associating with other females in similar reproductive states. For example, Möller et al. [35] found that reproductive country seemed to influence associations between female Indo-Pacific bottlenose dolphins (Tursiops aduncus), where females with same aged calves within social clusters usually exhibited strong association coefficient.
3.three. Dispersal Patterns
Our results revealed that in natural mother-offspring groups, male calves were all <2 years old (0.iii–ane.7 years old), whereas the female calves ranged from 0.one to nine.5 years of age. Additionally, three male calves not found in groups with their mothers were all older than two years (6.8-year-sometime 2009M14, 5.iv-year-old 2015M8, and 3.7-year-old 2011M6). Furthermore, ii mother-male person offspring pairs found trapped in the Yangtze mainstream in January 2014 had also been identified every bit mothers with their male calves younger than 2 years old (0.58-year-old and 0.75-twelvemonth-old, respectively; Ding Wang, unpublished information). This discrepancy could effect if male calves disperse from their natal groups at approximately ii years one-time, or at least are non in tight clan with their mothers as they may take been at nether two years sometime. Female person offspring might associate with their mothers at an older historic period, and can reproduce or mayhap render to reproduce in the natal grouping. Still, this association may not be strict as female offspring also emigrate out and raise offspring alone. For example, in this study we found twelve mother-calf pairs that remained lonely.
4. Materials and Methods
4.i. Study Location and Distribution of Porpoises
To investigate the health and social construction of the YFP population living in Poyang Lake, 4 capture-release surveys were conducted in the spring of 2009–2011, and 2015 (20–24 Feb 2009; 2–11 March 2010; 21–25 February 2011; and xi–20 March 2015), during the dry season of the lake. During this flavour, Poyang Lake is reduced to a set of channels together with dispersed sandpit areas (Figure 2). In this flavour, porpoises mainly distribute along the master channel between Hukou and Kangshan, and as well in some large sandpit areas betwixt Duchang and Yongxiu (Figure ii). Since those sandpits are connected to the main channel by shallow waters (depth ordinarily ≤2 g), some porpoises are restricted to those sandpit areas during the entirety of the dry out season. In 2009 and 2010, capture-release surveys were conducted in the channel of Duchang County, and in 2011 and 2015, the surveys were conducted in the large sandpit areas located between Duchang and Yongxiu (Figure 2).
4.two. Sample Collection
The well-adult "sound chase and net capture" method, which had been specially developed by the Institute of Hydrobiology of the Chinese University of Sciences to capture porpoises in the Yangtze main stream or natural reserves, was utilized to capture porpoises in the channel of Poyang Lake during the dry season. Once a group of porpoises (usually ≥2 individuals) was observed by the searching boat (a speed boat equipped with a 40 horsepower engine), chasing boats (consisting of 10–12 angling boats, each virtually 12 m long equipped with a 15 horsepower engine) would subsequently arrange themselves in line or curve and move in synchrony keeping a altitude of about 50 k to generate underwater racket and form an invisible audio barrier that would forcefulness the animals swimming slowly to a shallow water near the shore. Afterward, 2 internet boats (driving either face-to-face or in the opposite direction) would quickly release large-meshed nets to form a large enclosure (about 1 km2) to surround the porpoises. The porpoises would and so be driven to a smaller area with a radius of approximately 100 grand, where small-meshed capture nets would be released quickly to surround the porpoises. All animals were allowed to swim freely in the small enclosure to have plenty rest before whatever farther manipulation. After approximately thirty min, the fishermen would draw the capture nets slowly to shrink the enclosure until the animals were caught safely. Conversely, because the porpoises were restricted to a relatively small and shallow area (<1 km2) in the sandpit areas, the sound chasing operation was rendered unnecessary, leaving merely net capture protocols to exist used to take hold of the animals.
In each capture performance, only a single or a modest group of porpoises were captured. Later on the porpoises were successfully caught and sent to the examination platform, gender was identified, and then each male person or female was given a serial ID number (e.thou., 2009M1–2009M21, 2009F–2009F8) before proceeding. The series ID numbers of those porpoises that had been captured in the same net were recorded. If an adult female and a calf had been captured in the same net, a suspected mother-calf pair was then recorded. Reproductive state (pregnancy or lactation) of the developed female was too noted. Body weight and length were measured and recorded. Blood samples were as well drawn from the vein in the fluke using a dispensable syringe. Claret was anti-coagulated with acid-citrate-dextrose (ACD), and then preserved in liquid nitrogen until DNA extraction. A total of 132 individuals (including 10 recaptured individuals) were captured and sampled: 29 in 2009, 22 in 2010, 46 in 2011 and 35 in 2015. All captured individuals were marked using a unique internal passive intergrated tag label (PIT label; HT850, Hongteng Company, Guangzhou, China), which could exist identified via scanning. Group size and individual interaction data were all recorded during capturing (for details run across Table S1).
All capture-release surveys were authorized by the Poyang Lake Fishery and Fishing Administration Office of Jiangxi Province. All sampling was conducted in accordance with the Regulations of the People's Republic of Red china for the Implementation of Wild Aquatic Animal Protection (promulgated in 1993), and adhering to all upstanding guidelines and legal requirements in Red china.
4.3. DNA Extraction and PCR Distension
Genomic DNA was isolated using the Whole Genome DNA Extraction Kit (SBS, Shanghai, People's republic of china) following the manufacturer's instructions. Twenty-one polymorphic microsatellite loci (uncomplicated sequence repeats, SSR) were then used in parentage identification. Markers used included SSR1, SSR5, SSR8, SSR15, SSR22, SSR40, SSR41, SSR42, SSR51, SSR59, SSR63, SSR69, SSR71, SSR73, and SSR75 from Neophocaena phocaenoides asiaeorientalis [36,37,38]; PPHO130 from Phocoena phocoena [39], and NP391, NP404, NP409, NP464, and NP428 from Neophocaena phocaenoides [40,41]. PCR was performed in xv-μL reaction volumes containing 1 μL of template DNA, 1.5 μL 10× buffer, 0.7 μM of each primer, 0.25 mM deoxynucleotides (dNTPs), and 0.2 U of Taq DNA polymerase (Biostar; Wuhan Tianyuan Huida Biotech Company, Red china). Amplifications were carried out with atmospheric condition consisting of 95 °C for five min, followed by 33 cycles of denaturation at 95 °C for 30 south, annealing at 59.5 °C for 30 s and extension at 72 °C for 30 s, with a concluding extension step at 72 °C for 5 min. PCR products were separated by capillary electrophoresis on an ABI3130XL automated sequencer (Applied Biosystems, Foster City, CA, USA) and alleles were sized against the internal size standard (GeneScan ROX 500, ThermoFisher Scientific, Shanghai, Mainland china) using GeneMapperID v3.2 (Applied Biosystems). To minimize scoring error, samples that were homozygous, had low frequency alleles (only appeared in one or 2 individuals), or exhibited stutter bands, were amplified and genotyped at least three times. Nosotros used MICRO-CHECKER version 2.two.3 (Norwich Inquiry Park, Norwick, UK) [42] to cheque for null alleles, stuttering error, and allele dropout for each locus, at a confidence level of 95%.
A mtDNA control region segment of 597 bp, located at 84–680 bp of the complete control region, was selected due to highly variability [43]. The sequence was amplified with a forward primer (five′-GAA TTC CCC GGT CTT GTA AAC C-three′) and a reverse primer (5′-GGT TTG GGC CTC TTT GAG AT-3′) [44]. PCR amplifications were carried out in 25 μL reactions containing 10–100 ng genomic DNA, 0.half-dozen μM of each primer, 2.5 μL 10× buffer, 0.25 mM dNTPs, and ane U of Taq Deoxyribonucleic acid polymerase (Biostar). Amplifications startedat 95 °C for v min, followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at lx °C for 45 s, and extension at 72 °C for 90 due south, with a last extension stride at 72 °C for 7 min. PCR products were then purified using a purification kit (PCR Product Purification Kit, BioTeke, Beijing, China) and sequenced in both directions using the PCR primers. Sequencing was performed on an ABI3130 DNA sequencer (Applied Biosystems).
4.4. Data Analysis
We assessed measures of genetic diversity of microsatellite loci, including N a, H o, H due east, and PIC [45,46], using CERVUS version iii.0 (Field Genetics Ltd., London, UK) [47,48]. The N east-1p and N e-2p for each microsatellite locus, and the combined values of microsatellite loci for Due north e-1p and N e-2p, were calculated by using CERVUS version 3.0 [47,48,49]. Hardy-Weinberg equilibrium beyond all microsatellite loci was assessed via an exact probability test implemented in GENEPOP version 4.0 (Laboratiore de Genetique et Surround, Montpellier, France) [50]. FSTAT version two.9.3.2 (University of Lausanne, Lausanne, Switzerland) was used to summate the inbreeding coefficient alphabetize F is [51].
4.five. Detecting Potential Parents
We used the age-length formula established by Zhang [52] to summate the historic period of each individual. The human relationship between age (x) and body length (in cm) of male person (L g) and female (L f) YFP tin can be calculated equally follows:
50 m = 114.4458 x 0.1410 (♂ ≤ thirteen.0 years)
and
L f = 116.2519 x 0.0947 (♀ ≤ 16.5 years)
Although the age at get-go reproduction in females remains unknown, Zhang [52] and Wu et al. [53] estimated that YFP females are sexually mature at approximately four years of age and males at 4.five years of historic period. In order to non miss potential parents, we considered all female and male porpoises with calculated historic period ≥3 years old equally potential parents. Accordingly, we considered individuals iii years younger than the oldest within the population to be potential offspring.
four.six. Parentage Analysis
Parentage analysis was conducted using the maximum likelihood (ML) method implemented in program CERVUS version three.0 [47,48]. This method compares the likelihood of the two most likely mothers or fathers. For each offspring, the difference between the likelihoods of the two about probable mothers or fathers produces a Δ score. Simulations were conducted to estimate the critical values of Δ required to assign parentage with a certain caste of confidence, based on the assumptions made nigh the population. Parentage assigned at both the 95% and lxxx% confidence levels were reported, equally determined by the critical Δ score. We gear up parameters for the simulation as follows: the proportion of loci was i.0, the proportion of potential father and mother were both 20%, the level of potential mistyping was 1%. Simulations were conducted for 100,000 repetitions.
The r was also calculated to analyze kinship. The theoretical values between parent-offspring and total-sibs are 0.5; those betwixt half-sibs are 0.25 [21,54]. To calculate r estimates, we used the triadic likelihood reckoner (TrioML [55]). This estimator computes the relatedness of a dyad in relation to a tertiary reference private in guild to minimize errors stemming from identity-in-land rather than identity-past-descent. It further allows the specification of a genotyping error rate and is bounded between 0 and 1, a more legitimate range than that of other estimators. An evaluation using empirical and simulated data for seven unlike estimators showed that the TrioML figurer produced the most accurate estimates of kinship [55].
5. Conclusions
In this study, group composition and dispersal patterns were studied for the YFP population living in Poyang Lake. To avert homo disturbance to natural groups and guarantee the reliability of the results, we identified natural groups based on both genetic parentage and observational data. Results indicated maternal relationship and reproductive condition may be important factors for group composition of females. The dispersal patterns of the YFP showed that male person calves may disperse from their mothers at approximately ii years former, or at least they were not in tight association with their mothers every bit they may have been under 2 years one-time. Female offspring are observed to stay longer with their mothers and can reproduce in the natal grouping.
In the wild, the YFP is difficult to place and track, and samples used for relatedness studies are hard to obtain. Therefore, social beliefs studies for this population in detail are poor. This study collected information from iv capture-release surveys, however, accurate and valuable information is still restricted. For case, calculated parentage was limited, which may exist because of the potential high mortality of calves in the Poyang Lake during the dry out season, ultimately reducing detectable aspects of social behavior in this study. In addition, although nosotros estimated the dispersal window of male calves, these observations were also limited to few datum points and they should be prudently treated.
Supplementary Materials
Acknowledgments
We would like to thank our staff for collecting samples. We thank Wang Jinliang (Constitute of Zoology, Zoological Society of London) for his kind help with the relatedness analysis. We too thank Kathryn Stewart for proofreading the revision. This piece of work was supported by grants from the Special Fund for Agro-scientific Research in the Public Involvement (No. 201203086) to Ding Wang, Jinsong Zheng and Yujiang Hao; the National Natural Science Foundation of China (No. 31430080 to Ding Wang, and No. 31000168 to Jinsong Zheng) and the Knowledge Innovation Program of Chinese University of Sciences (No. KSCX2-EW-Z-4) to Ding Wang. This piece of work was also supported past Major Project of Natural Science Research in Anhui Province (No. KJ2016A863) to Minmin Chen.
Author Contributions
Minmin Chen conducted the study, analyzed information and wrote the manuscript. Jinsong Zheng and Ding Wang designed the study and revised the manuscript. Yang Zheng, Yujiang Hao and Zhigang Mei conducted the written report and supplied assistance. Kexiong Wang directed the capture-release surveys and revised the manuscript. Qingzhong Zhao helped collect samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mann, J. Cetacean Societies: Field Studies of Dolphins and Whales; University of Chicago Press: Chicago, IL, U.s., 2000. [Google Scholar]
- Michaud, R. Sociality and Ecology of the Odontocetes. In Sexual Segregation in Vertebrates: Ecology of the Ii Sexes; Cambridge University Press: Cambridge, UK, 2005; pp. 303–326. [Google Scholar]
- Möller, 50.M.; Beheregaray, L.B. Genetic prove for sex-biased dispersal in resident bottlenose dolphins (Tursiops aduncus). Mol. Ecol. 2004, thirteen, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Karczmarski, Fifty.; Würsig, B.; Gailey, K.; Larson, K.Westward.; Vanderlip, C. Spinner dolphins in a remote Hawaiian atoll: Social group and population structure. Behav. Ecol. 2005, sixteen, 675–685. [Google Scholar] [CrossRef]
- Baird, R.W.; Whitehead, H. Social organization of mammal-eating killer whales: Group stability and dispersal patterns. Can. J. Zool. 2000, 78, 2096–2105. [Google Scholar] [CrossRef]
- Ortega-Ortiz, J.M.; Engelhaupt, D.; Winsor, M.; Mate, B.R.; Rus Hoelzel, A. Kinship of long-term associates in the highly social sperm whale. Mol. Ecol. 2012, 21, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Mirimin, L.; Banguera-Hinestroza, E.; Dillane, E.; Hoelzel, A.R.; Cross, T.F.; Rogan, Due east. Insights into genetic diversity, parentage, and group composition of Atlantic white-sided dolphins (Lagenorhynchus acutus) off the due west of Ireland based on nuclear and mitochondrial genetic markers. J. Hered. 2011, 102, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Reeves, R.R. River cetaceans and habitat change: Generalist resilience or specialist vulnerability? J. Mar. Biol. 2012, 2012, 1–xi. [Google Scholar] [CrossRef]
- Gao, A.; Zhou, M. Geographical variation of external measurements and three subspecies of Neophocaena phocaenoides in Chinese waters. Acta Theriol. Sin. 1995, fifteen, 81–92. [Google Scholar]
- Mei, Z.; Huang, Due south.50.; Hao, Y.; Turvey, S.T.; Gong, Westward.; Wang, D. Accelerating population refuse of Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis). Biol. Conserv. 2012, 153, 192–200. [Google Scholar] [CrossRef]
- Wang, D.; Turvey, S.T.; Zhao, 10.; Mei, Z. Neophocaena asiaeorientalis ssp. asiaeorientalis. In IUCN Red List of Threatened Species Version 2013.i; Available online: http://www.iucnredlist.org/ (accessed on 2 September 2013).
- Taylor, A.; Horsup, A.; Johnson, C.; Sunnucks, P.; Sherwin, B. Relatedness structure detected past microsatellite analysis and attempted full-blooded reconstruction in an endangered marsupial, the northern hairy-nosed wombat Lasiorhinus krefftii. Mol. Ecol. 1997, 6, nine–19. [Google Scholar] [CrossRef] [PubMed]
- Onorato, D.P.; Hellgren, Eastward.C.; Van Den Bussche, R.A.; Skiles, J.; Raymond, J. Paternity and relatedness of American black bears recolonizing a desert montane island. Can. J. Zool. 2004, 82, 1201–1210. [Google Scholar] [CrossRef]
- Itoh, T.; Sato, Y.; Kobayashi, K.; Mano, T.; Iwata, R. Effective dispersal of brown bears (Ursus arctos) in eastern Hokkaido, inferred from analyses of mitochondrial DNA and microsatellites. Mamm. Study 2012, 37, 29–41. [Google Scholar] [CrossRef]
- Zhang, Ten.; Liu, R.; Zhao, Q.; Zhang, Thousand.; Wei, Z.; Wang, X.; Yang, J. The population of finless porpoise in the heart and lower reaches of Yangtze River. Acta Theriol. Sin. 1993, 13, 260–270. [Google Scholar]
- Wei, Z.; Wang, D.; Zhang, X.; Zhao, Q.; Wang, Thou.; Kuang, X.A. Population size, behavior, motility pattern and protection of Yangtze finless porpoise at Balijiang department of the Yangtze River. Resour. Environ. Yangtze Basin 2002, 11, 427–432. [Google Scholar]
- Yang, J.; Chen, P. Movement and behavior of finless porpoise (Neophocaena phocaenoides) at Swan Oxbow, Hubei province. Acta Hydrobiol. Sin. 1996, 20, 32–40. [Google Scholar]
- Jiang, W. Observation on the group of the ChangJiang finless porpoise conserved in semi-nature weather. J. Anhui Univ. (Nat. Sci.) 2000, 24, 106–111. [Google Scholar]
- Wei, Z.; Wang, D.; Zhang, X. Assemblage and spatio-temporal distribution of the Yangtze finless porpoise Neophocaena phocaenoides asiaeorientalis in Tian-E-Zhou National Baiji Reserve. Acta Hydrobiol. Sin. 2004, 28, 247–252. [Google Scholar]
- Mei, Z.; Zhang, X.; Huang, Southward.-L.; Turvey, S.T.; Gong, W.; Wang, D. The Yangtze finless porpoise: On an accelerating path to extinction? Biol. Conserv. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Csilléry, K.; Johnson, T.; Beraldi, D.; Clutton-Brock, T.; Coltman, D.; Hansson, B.; Spong, G.; Pemberton, J.Grand. Performance of marker-based relatedness estimators in natural populations of outbred vertebrates. Genetics 2006, 173, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Blouin, Thou.; Parsons, M.; Lacaille, V.; Lotz, South. Use of microsatellite loci to classify individuals past relatedness. Mol. Ecol. 1996, five, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Isberg, South.; Chen, Y.; Barker, South.; Moran, C. Assay of microsatellites and parentage testing in saltwater crocodiles. J. Hered. 2004, 95, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Haanes, H.; Rosef, O.; Veiberg, V.; Roed, 1000.H. Microsatellites with variation and heredity applicative to genetic studies of Norwegian cherry deer (Cervus elaphus atlanticus). Anim. Genet. 2005, 36, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Cronin, Grand.; Shideler, R.; Hechtel, J.; Strobeck, C.; Paetkau, D. Genetic relationships of grizzly bears (Ursus arctos) in the Prudhoe Bay region of Alaska: Inference from microsatellite DNA, mitochondrial DNA, and field observations. J. Hered. 1999, 90, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Cronin, Grand.A.; Shideler, R.; Waits, 50.; Nelson, R.J. Genetic variation and relatedness in grizzly bears in the Prudhoe Bay region and side by side areas in northern Alaska. Ursus 2005, 16, 70–84. [Google Scholar] [CrossRef]
- Mirimin, L.; Andrew, W.; Emer, R.; Patricia, R.; Andrew, R.; Jame, C.; Tom, C. Population structure of brusk-beaked common dolphins (Delphinus delphis) in the Due north Atlantic Body of water as revealed by mitochondrial and nuclear genetic markers. Mar. Biol. 2009, 156, 821–834. [Google Scholar] [CrossRef]
- Chen, L.; Bruford, 1000.Westward.; Xu, Due south.; Zhou, M.; Yang, G. Microsatellite variation and significant population genetic structure of endangered finless porpoises (Neophocaena phocaenoides) in Chinese coastal waters and the Yangtze River. Mar. Biol. 2010, 157, 1453–1462. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.; Gong, C.; Zhao, Q.; Wang, D. Inbreeding evaluation on the ex situ conserved Yangtze finless porpoise population in Tian'ezhou national natural reserve. Chin. J. Zool. 2014, 49, 305–316. [Google Scholar]
- Fullard, 1000.; Early, Thousand.; Heide-Jørgensen, Grand.; Bloch, D.; Rosing-Asvid, A.; Amos, Westward. Population structure of long-finned pilot whales in the Northward Atlantic: A correlation with sea surface temperature? Mol. Ecol. 2000, 9, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Palsbøll, P.J.; Heide-Jørgensen, G.P.; Berubé, G. Analysis of Mitochondrial Control Region Nucleotide Sequences from Baffin Bay Belugas (Delphinapterus leucas): Detecting Pods or Sub-Populations? In Belugas in the North Atlantic and Russian Arctic Tromsø: N Atlantic Marine Mammal Commission; Heide-Jørgensen, M.P., Wiig, Ø., Eds.; NAMMCO Scientific Publications: Nuuk, Greenland, Kingdom of denmark, 2002; pp. 39–fifty. [Google Scholar]
- Whitehead, H. Sperm Whales: Social Evolution in the Ocean; Chicago Academy Press: Chicago, IL, The states, 2003. [Google Scholar]
- Bigg, Grand.A.; Olesiuk, P.F.; Ellis, Thou.M.; Ford, J.K.B.; Balcomb, One thousand.C. Social organization and genealogy of resident killer whales (Orcinus orca) in the littoral waters of British Columbia and Washington State. Rep. Int. Whal. Commun. 1990, 12, 383–405. [Google Scholar]
- Gowans, Due south.; Würsig, B.; Karczmarski, L. The social structure and strategies of delphinids: Predictions based on an ecological framework. Adv. Mar. Biol. 2008, 53, 195–294. [Google Scholar]
- Möller, L.K.; Beheregaray, L.B. Coastal bottlenose dolphins from southeastern Commonwealth of australia are Tursiops aduncus co-ordinate to sequences of the mitochondrial Deoxyribonucleic acid control region. Mar. Mamm. Sci. 2011, 17, 249–263. [Google Scholar] [CrossRef]
- Zheng, J.Due south.; Liao, X.50.; Tong, J.G.; Du, H.J.; Milinkovitch, M.C.; Wang, D. Development and characterization of polymorphic microsatellite loci in the endangered Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis). Conserv. Genet. 2008, nine, 1007–1009. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, J.; Zhou, Z.; Lin, Yard.; Wang, D.; Zheng, B.; Jiang, W. Paternity conclusion of captivity-bred Yangtze finless porpoises Neophocaena phocaenoides asiaeorientalis past microsatellite genotyping. Prog. Mod. Biomed. 2009, 9, 4015–4020. [Google Scholar]
- Zhou, Z.; Zheng, J.S.; Chen, Thou.M.; Zhao, Q.Z.; Wang, D. Genetic Evaluation and Evolution Prognosis on Ex Situ Conserved Yangtze Finless Porpoise Living in Tian-due east-Zhou National Natural Reserve. Acta Hydrobiol. Sin. 2012, 36, 403–411. [Google Scholar]
- Rosel, P.; France, South.; Wang, J. Genetic construction of harbour porpoise Phocoena phocoena populations in the northwest Atlantic based on mitochondrial and nuclear markers. Mol. Ecol. 1999, 8, S41–S54. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bruford, Grand.; Yang, G. Isolation and characterization of microsatellite loci in the finless porpoise (Neophocaena phocaenoides). Mol. Ecol. Notes 2007, 7, 1129–1131. [Google Scholar] [CrossRef]
- Chen, 50.; Yang, Yard. Development of tetranucleotide microsatellite loci for the finless porpoise (Neophocaena phocaenoides). Conserv. Genet. 2008, nine, 1033–1035. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. Micro-checker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, four, 535–538. [Google Scholar] [CrossRef]
- Zheng, J.S.; Xia, J.H.; He, Southward.P.; Wang, D. Population genetic structure of the Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis): Implications for management and conservation. Biochem. Genet. 2005, 43, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zheng, J.; Wu, M.; Ruan, R.; Zhao, Q.; Wang, D. Genetic variety and population construction of the critically endangered Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) equally revealed by mitochondrial and microsatellite Deoxyribonucleic acid. Int. J. Mol. Sci. 2014, 15, 11307–11323. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.50.; Skolnick, Chiliad.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar] [PubMed]
- Hearne, C.K.; Ghosh, South.; Todd, J.A. Microsatellites for linkage analysis of genetic traits. Trends Genet. 1992, 8, 288–294. [Google Scholar] [CrossRef]
- Marshall, T.; Slate, J.; Kruuk, Fifty.; Pemberton, J. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping mistake increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Taylor, C. Comparisons of iii probability formulae for parentage exclusion. Anim. Genet. 1997, 28, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F. GENEPOP'007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT 2.nine.iii.2, a Plan to Judge and Test Cistron Diversities and Fixation Indices. Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 23 February 2002).
- Zhang, X. Studies on the age conclusion, growth and reproduction of finless porpoise Neophocaena phocaenoides. Acta Hydrobiol. Sin. 1992, 16, 289–298. [Google Scholar]
- Wu, H.P.; Hao, Y.J.; Li, X.; Zhao, Q.Z.; Chen, D.Q.; Kuang, X.A.; Kou, Z.B.; Feng, 1000.K.; Gong, W.Yard.; Wang, D.B. Mode ultrasonographic evaluation of the testis in relation to serum testosterone concentration in male Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis) during the convenance flavour. Theriogenology 2010, 73, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Queller, D.C.; Goodnight, K.F. Estimating relatedness using genetic markers. Development 1989, 43, 258–275. [Google Scholar] [CrossRef]
- Wang, J. Triadic IBD coefficients and applications to estimating pairwise relatedness. Genet. Res. 2007, 89, 135–153. [Google Scholar] [CrossRef] [PubMed]
Figure 1. Twenty-one mother-offspring pairs and six father-offspring pairs detected by CERVUS in the Yangtze finless porpoise population living in Poyang Lake. Parent-offspring pairs in dotted boxes were natural groups. The maternal line consisting of three generations was a natural maternal group captured in a sandpit in 2011. Blue represents offspring younger than two years old. Orangish stands for offspring older than two years old. Pairwise relatedness alphabetize r was calculated past the triadic likelihood estimator (TrioML).
Figure 1. Twenty-i mother-offspring pairs and half dozen father-offspring pairs detected past CERVUS in the Yangtze finless porpoise population living in Poyang Lake. Parent-offspring pairs in dotted boxes were natural groups. The maternal line consisting of iii generations was a natural maternal grouping captured in a sandpit in 2011. Blue represents offspring younger than two years old. Orange stands for offspring older than ii years old. Pairwise relatedness index r was calculated past the triadic likelihood reckoner (TrioML).
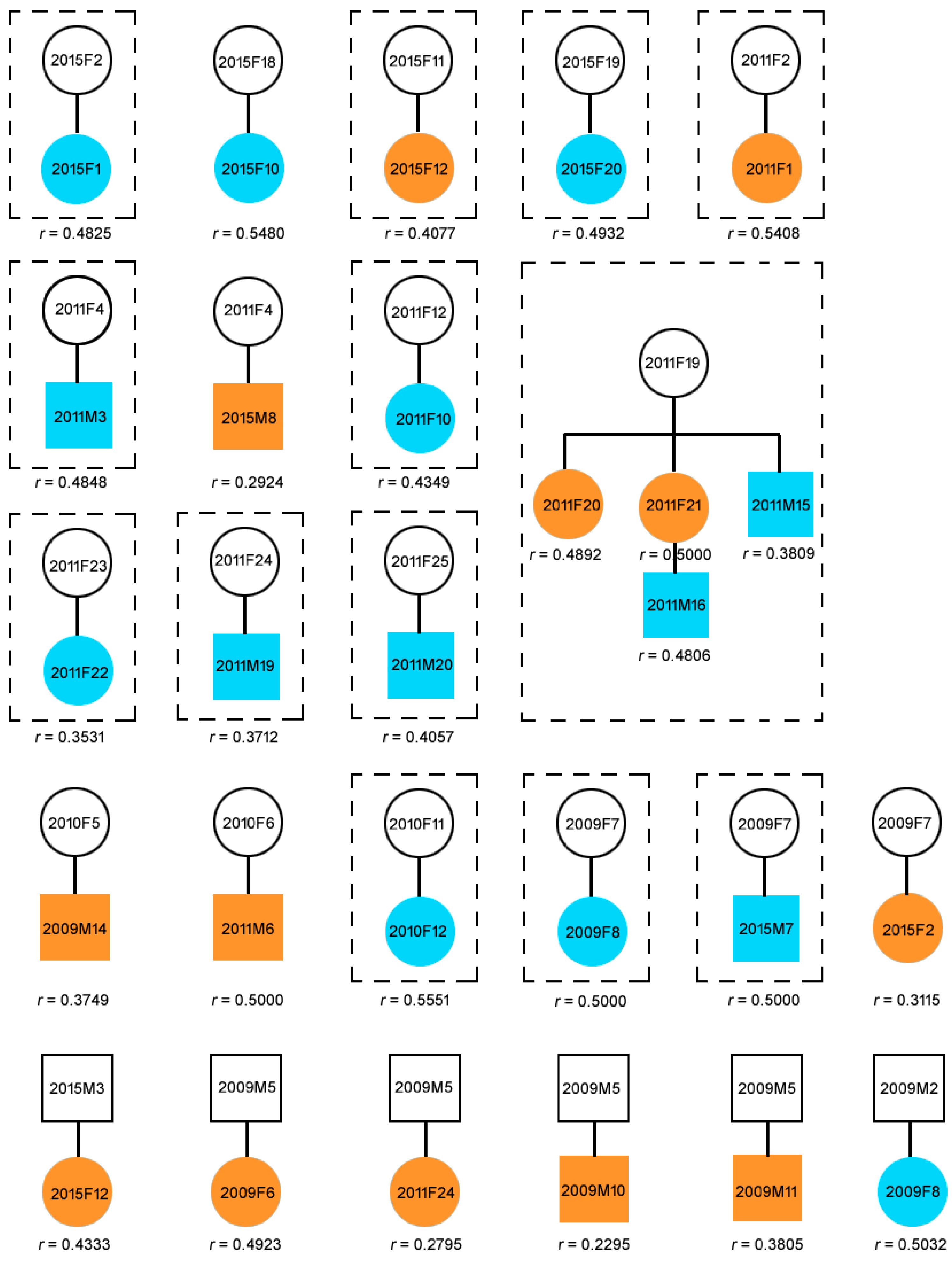
Figure ii. Water coverage of the Poyang Lake in the early spring. Porpoises primarily distribute forth the main aqueduct betwixt Hukou and Kangshan, and also in some large sandpit areas between Duchang and Yongxiu. The bluish box represents the sampling expanse in 2009 and 2010. The ruddy circle represents the sampling surface area in 2011 and 2015.
Figure 2. H2o coverage of the Poyang Lake in the early spring. Porpoises primarily distribute forth the main channel between Hukou and Kangshan, and too in some large sandpit areas between Duchang and Yongxiu. The blue box represents the sampling expanse in 2009 and 2010. The red circumvolve represents the sampling area in 2011 and 2015.
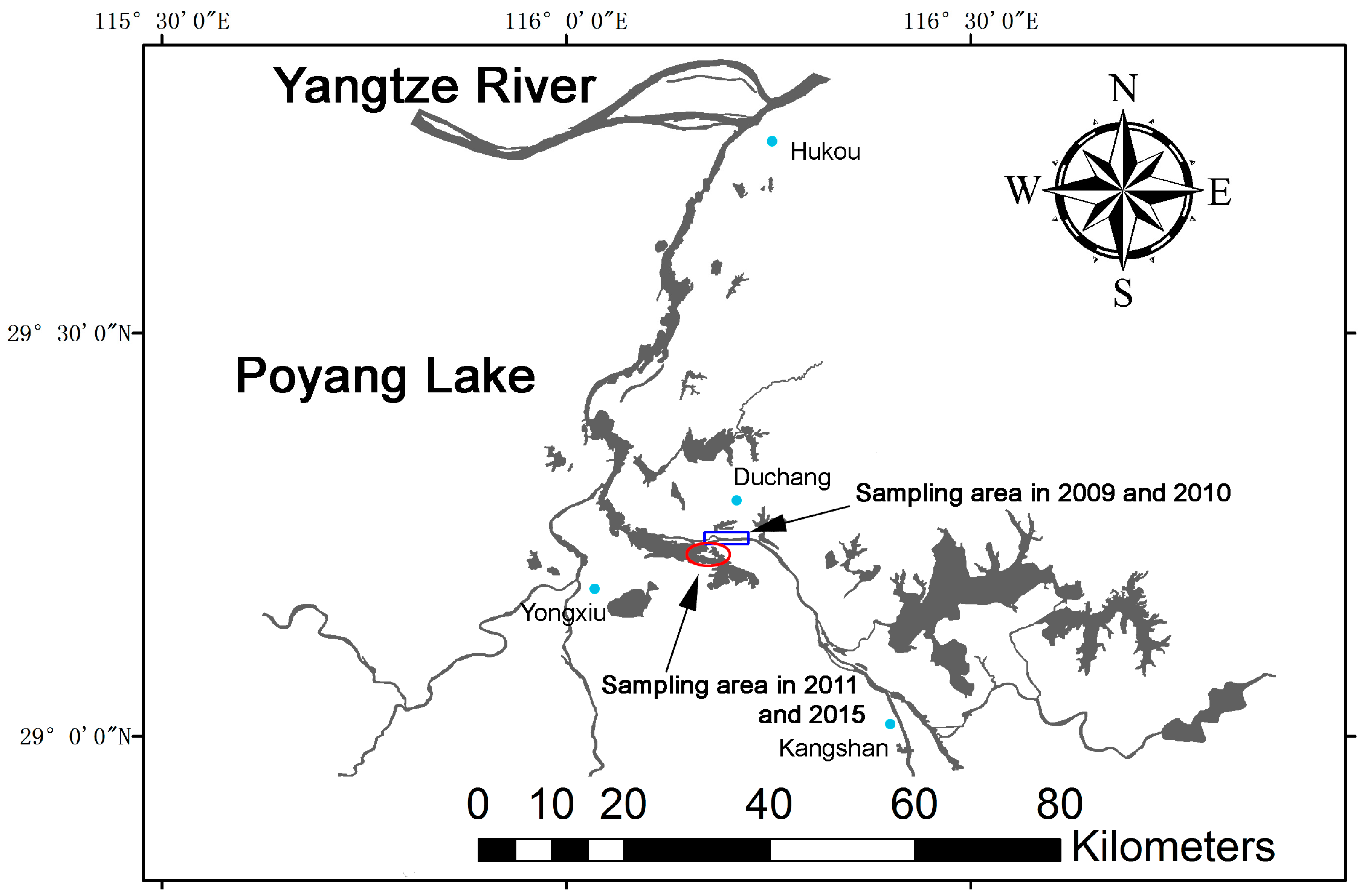
Table one. Characteristics of genetic diversity at 21 microsatellite loci for 122 Yangtze finless porpoises in the Poyang Lake.
| Locus | North a | H o | H e | PIC | N e-1p | Due north e-2p | F is |
|---|---|---|---|---|---|---|---|
| SSR1 | half dozen | 0.639 | 0.629 | 0.567 | 0.785 | 0.629 | −0.017 |
| SSR5 | 9 | 0.795 | 0.799 | 0.765 | 0.580 | 0.402 | 0.005 |
| SSR8 | 6 | 0.800 | 0.740 | 0.691 | 0.678 | 0.503 | −0.082 |
| SSR15 | xi | 0.746 | 0.716 | 0.660 | 0.708 | 0.541 | −0.041 |
| SSR22 | 4 | 0.689 | 0.665 | 0.591 | 0.777 | 0.628 | −0.041 |
| SSR40 | 10 | 0.746 | 0.746 | 0.705 | 0.657 | 0.477 | 0.000 |
| SSR41 | 6 | 0.425 | 0.673 | 0.627 | 0.736 | 0.561 | 0.009 |
| SSR42 | v | 0.610 | 0.671 | 0.608 | 0.751 | 0.592 | 0.092 |
| SSR51 | 12 | 0.780 | 0.796 | 0.765 | 0.574 | 0.396 | 0.017 |
| SSR59 | ten | 0.854 | 0.828 | 0.801 | 0.522 | 0.349 | −0.031 |
| SSR63 | 7 | 0.742 | 0.632 | 0.559 | 0.787 | 0.641 | −0.182 |
| SSR69 | seven | 0.694 | 0.611 | 0.575 | 0.784 | 0.606 | −0.136 |
| SSR71 | 7 | 0.529 | 0.523 | 0.490 | 0.848 | 0.682 | −0.013 |
| SSR73 | 6 | 0.782 | 0.793 | 0.756 | 0.599 | 0.420 | 0.013 |
| SSR75 | 16 | 0.672 | 0.644 | 0.625 | 0.733 | 0.54 | −0.044 |
| NP391 | 12 | 0.415 | 0.475 | 0.441 | 0.877 | 0.723 | 0.128 |
| NP404 | 5 | 0.504 | 0.555 | 0.459 | 0.844 | 0.736 | 0.077 |
| NP409 | 9 | 0.664 | 0.748 | 0.706 | 0.652 | 0.476 | 0.113 |
| NP428 | 5 | 0.374 | 0.361 | 0.345 | 0.930 | 0.792 | −0.035 |
| NP464 | 9 | 0.628 | 0.764 | 0.724 | 0.634 | 0.456 | 0.178 |
| PPHO130 | 9 | 0.795 | 0.787 | 0.752 | 0.596 | 0.419 | −0.010 |
| Mean of 21 loci | 8.i | 0.661 | 0.674 | 0.629 | Combined 7.20 × 10−4 | Combined ii.17 × 10−half dozen | 0.0002 (p > 0.05) |
© 2016 past the authors; licensee MDPI, Basel, Switzerland. This commodity is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-By) license (http://creativecommons.org/licenses/past/4.0/).
What Should Be The Cut Off Of Inbreeding Coefficient In Registered Angus,
Source: https://www.mdpi.com/1422-0067/17/8/1268/htm
Posted by: allenleareved.blogspot.com

 ,
, 
0 Response to "What Should Be The Cut Off Of Inbreeding Coefficient In Registered Angus"
Post a Comment